BayCare Provides Access to Numerous Testing Options for COVID-19
As COVID-19 continues to spread across the Tampa Bay area, the need for testing remains at an all-time high.
But not all tests are the same. Different manufacturers and laboratories have different chemical recipes and technology platforms for test processing; providers can collect specimens in different ways which can impact results; and where and how tests are processed can greatly impact the time it takes to get results.
As the scientific community is getting to know the novel coronavirus better, testing protocols are being refined and new options for testing are emerging that are expected to play a bigger role in how communities respond to the pandemic.
“So much has changed, just in the four months since BayCare opened its first drive-thru testing site,” said Jim Cote, senior vice president for ambulatory services. “Our goal is to keep expanding our community’s access to testing so that those who test positive can isolate early and help prevent the virus from spreading to others.”
Because no test platform has the supplies and the processing capacity to meet the demand for all COVID-19 testing, BayCare and other health care providers are using a variety of manufacturers’ platforms and processing options to provide as much capacity as possible. BayCare is only using tests cleared by the U.S. Food and Drug Administration with Emergency Use Authorization (EUA). And before using them for any patients, BayCare’s laboratories also validate the process to make sure the tests perform as claimed. Learn more, here.
“COVID-19 testing is complex and the laboratory community is still learning about its intricacies,” said Maura Pieretti, PhD, scientific director for BayCare Laboratories. “However, here at BayCare we’ve made significant progress adding new methodologies, instruments and platforms to allow us to evolve and process tests quickly, safely and effectively.”
Generally, COVID-19 tests fall into three different categories based on the science they utilize: Nucleic Acid (or viral), Antibody and Antigen.
NUCLEIC ACID (VIRAL) TESTS
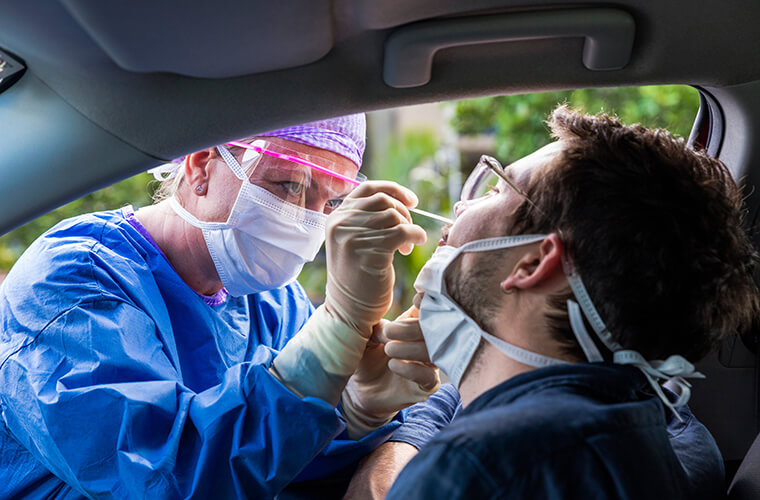
Nucleic acid (which is also known as viral) testing is the primary mechanism currently being used for COVID-19 diagnostic testing. The main amplification technology is called “polymerase chain reaction” (PCR) and uses reagent chemicals to make multiple copies of viral genes to rapidly determine if the virus is present.
Collection: Collection of a patient’s specimen is done by inserting a specific type of swab in the upper respiratory area. BayCare generally uses a 6-inch swab that reaches deep in the nasal cavity, touching the nasopharyngeal area. BayCare believes this location provides a better chance at finding viral infection and improve reliability of results. “Early in the disease, the upper body sites are a good source of the virus,” said Pieretti. “Later in the disease, the virus goes down deeper, and the nose swab may not be a good choice. The swab collection, technique and body site can cause a false negative result.”
The newly-available test-at-home kit provided by LabCorp and others use a less invasive short swab to collect a specimen in the nose or mouth. This makes it easier to conduct test collection and avoid the need for special test collection sites that use highly-skilled personnel, such as BayCare’s drive-thru sites. But the results may be less reliable as the viral load is believed to be usually lower in those areas of the body. And the risk of cross-contamination may also be higher since non-trained individuals may oversee the test collection.
“This option definitely helps expand access to testing,” said Cote. “This provides people with an easy and convenient way of getting tested from the comfort of their own home.”
Reliability: In general, a false COVID-19 positive is rare in viral testing – unless somehow the patient’s sample was contaminated during collection or at the laboratory, said Pieretti. False negatives are more common and can be impacted by all kinds of variables, including the body site where the specimen was collected (there may not be enough viral load present) or, most importantly, when in the infection cycle the patient is. And regardless, results are only applicable to the time of test collection. Patients can end up being exposed to the coronavirus anytime after the test.
“When the virus is in very low amounts, the tests can be less accurate,” said Pieretti. “A very small amount of virus can be harder to recognize and may generate a false negative result. Or, one may see a positive result, followed by a negative, then followed by another positive. This happens for example in patients that are recovering. We don’t know whether a positive result at these late stages correspond with the ability of the patient to spread the virus and infect others.”
Speed of results: Depending on the manufacturer and platform, PCR tests can take anywhere from a couple of hours to more than a day to receive results. Generally, health care providers such as BayCare have reserved “rapid-test” platforms for their hospital settings, so acutely ill patients can be diagnosed quickly and treated efficiently. BayCare processes most tests in-house, through BayCare Laboratories. When test demand is high, BayCare send specimens collected from drive-thru testing sites to commercial laboratories for processing. When a patient receives test results is also dependent on how long it takes a lab to process the samples, including patient registration and specimens transportation – which can significantly add to the turnaround times.
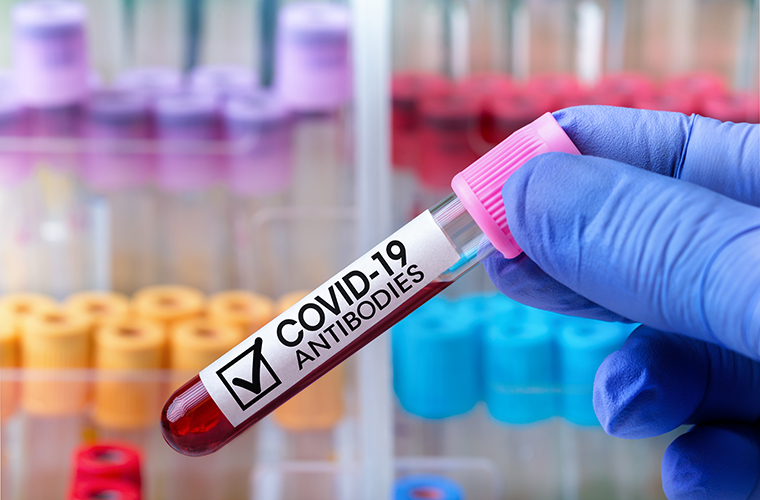
ANTIBODY TESTS
Antibody testing is used to learn if someone has had a past infection – it is NOT a diagnostic tool. An antibody test helps determine any presence of antibodies, which are specific proteins the body produces to fight off a COVID-19 infection. Scientists still don’t know if the presence of antibodies help protect the patients from being infected a second time, but it does identify individuals to help in the donation of convalescent plasma for current COVID-19 patients. At BayCare, patients are required to have a doctor’s referral to obtain an antibody test.
For the plasma donations, donors must meet certain criteria to donate blood but do not need a doctor’s note. For more information, potential donors can contact OneBlood at 1-888-936-6283 and select option 9 or visit their website here.
Collection: The test requires a blood draw.
Reliability: Very high.
Speed of results: Test results from BayCare are available between 48 and 72 hours.
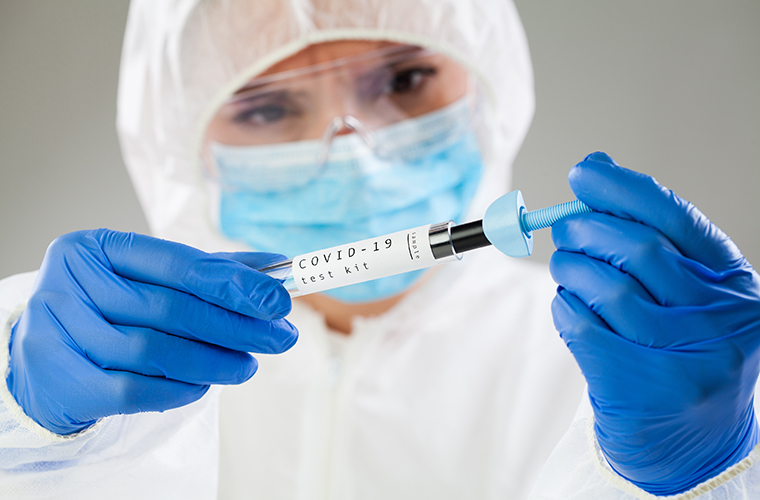
ANTIGEN TESTS
Soon, BayCare anticipates bringing antigen testing as an option in its doctor’s offices and urgent care centers. The antigen test helps detect if a person is infected by looking at proteins found on the surface of the virus. Similar to a strep throat platform, the technology to run antigen tests is far more ubiquitous and can be done onsite.
Collection: A commonly used medical swab collects a specimen from the nose or mouth.
Reliability: Good. False negatives can occur, but because of the inexpensive nature of the technology, a re-test for symptomatic patients would likely be common.
Speed of results: Almost immediate.
WHAT TEST IS RIGHT FOR YOU
To better understand what type of testing is appropriate for each patient – or whether you need to get one at all, BayCare offers various screening options including a Nurse Triage phone line (1-800-BayCare) and the Online Screening Tool available on BayCare’s website. These tools help guide patients to the proper care.
It’s important to note that hospitals are not designated COVID-19 testing sites. In-hospital testing is reserved for patients that need emergency care.
For more information, visit BayCare.org/Coronavirus.